[ad_1]
As drivers around the world switch to electric cars, new batteries that can store more energy, translating to longer driving distances before a car needs recharging, can’t come soon enough. But researchers at NTNU have discovered that one promising material in the search for the next generation of batteries needs rethinking.
Today, lithium ion batteries—the kind that are used in smartphones and other devices as well as electric cars—contain liquid electrolytes. Charged atoms known as ions move in one direction through the electrolyte, releasing an electron in order to power a device. To recharge the battery, ions move back through the electrolyte and regain an electron.
But batteries with liquid electrolytes are approaching their theoretical energy density limit. To store more energy without having to increase battery size, researchers are working on solid-state electrolytes instead. Solid-state batteries would be safer and more stable than their liquid electrolyte counterparts, making them useful in pacemakers and wearable devices, too.
Mixing ceramics with polymers
A polymer known as poly(ethylene oxide), or PEO, fits the bill as a solid electrolyte because it is flexible, light, and easy to process. But there’s a catch—PEO isn’t great at the one thing an electrolyte needs to do well: conduct lithium ions. Ceramic materials, on the other hand, conduct ions well but don’t have the mechanical advantages of polymers.
So researchers began adding ceramic powders to the polymer as a “filler” material. “The idea was to put the ceramic particles inside these polymers, and somehow enjoy the best of both worlds,” says Daniel Rettenwander, an associate professor in the Department for Materials Science and Engineering at NTNU. In fact, many publications report that polymer electrolytes conduct ions better after adding fillers, even suggesting that the fillers form fast-track networks for lithium ions to move through the material.
But in work published in the journal Frontiers in Energy Research, Rettenwander and colleagues have found that the fillers don’t actually play a part in transporting lithium ions in the electrolyte at all. While that may not sound like good news to those who were pinning their hopes on this simple combination of polymer and ceramic, the discovery could help nudge researchers down a more fruitful path that eventually leads to batteries we can use.
Understanding why it behaves the way it does
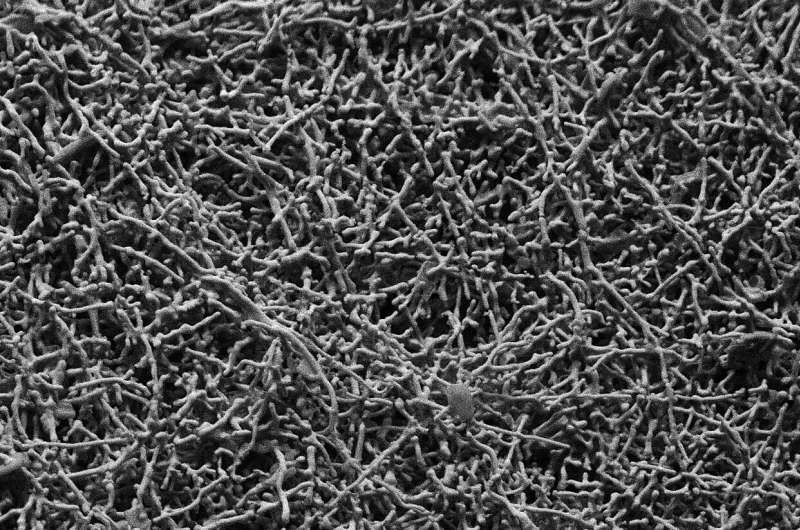
Rettenwander and colleagues made membranes with varying amounts of a lithium-oxide garnet ceramic filler known as LLZO, in the form of both particles and wires just nanometers in width. Then they looked at cross sections of the membranes using a scanning electron microscope and, in collaboration with Roland Brunner at the Materials Research Center in Leoben, Austria, used X-ray computer tomography to take snapshots of the inside of the materials at the microscale.
Comparing those snapshots with measurements of the material’s properties meant the researchers could draw conclusions about how the filler particles affect the behavior of ions in the polymer. “We can understand why it behaves like it behaves,” says Rettenwander.
The researchers saw that both the particles and nanowires were evenly distributed throughout the polymer, and didn’t form networks that could fast-track lithium ions. The membranes with more of the filler were actually worse conductors of the ions, bolstering the conclusion that the filler doesn’t take part in transporting the ions.
“Just putting filler inside the membrane doesn’t lead to any improvements,” says Rettenwander. “It’s not the intrinsic property of the ceramic which improves the performance.”
So why do some experiments find that adding fillers increases conductivity, if they’re not actually playing a part in transporting ions? Rettenwander thinks it could be the changes that occur in the polymer itself that give the materials their advantage—going from an orderly crystal structure to a more amorphous, irregular pattern.
Could almost double energy density
This doesn’t mean that polymers with added filler are a dead end for research on solid state batteries. “The use of fillers is still a very good strategy, but it’s just that it’s not enough to put the filler inside,” says Rettenwander. “You have to improve the interface between the polymer and the ceramic in order to make these membranes work.” He’s working on a way to help the materials bond better by changing their surfaces, for example.
If researchers can find a way to combine the best of both materials, solid state batteries for electric cars could significantly cut down on charging stops. “If you are able to make a solid state battery, then you will be able to almost double the energy density, increasing your driving distance almost by a factor of two,” says Rettenwander.
An in-situ generated composite solid-state electrolyte for high-voltage lithium metal batteries
Mir Mehraj Ud Din et al, Role of Filler Content and Morphology in LLZO/PEO Membranes, Frontiers in Energy Research (2021). DOI: 10.3389/fenrg.2021.711610
Citation:
Making materials for the next generation of electric car batteries (2022, April 26)
retrieved 30 April 2022
from https://techxplore.com/news/2022-04-materials-electric-car-batteries.html
This document is subject to copyright. Apart from any fair dealing for the purpose of private study or research, no
part may be reproduced without the written permission. The content is provided for information purposes only.
[ad_2]
Source link